How Bath mathematics research is tackling global health challenges

Introducing the Institute for Mathematical Innovation
The Institute for Mathematical Innovation (IMI) at the University of Bath was launched in October 2015 with a goal of turning data into decisions. Over the past decade, its team of mathematicians and statisticians have combined data analysis and mathematical modelling to support organisations in a range of sectors: from investigating techniques to remove bias from machine learning algorithms, to creating a model for researchers to understand how urban environments affect wellbeing in support of healthier urban planning.
Introducing the IMI
As a leading UK research institute in applied mathematics, the Institute adds value and impact to research by creating interdisciplinary connections through modelling, data science and machine learning. While IMI research spans themes such as environmental extremes and quantum technologies, its healthcare work is making a tangible impact.
Dr Laura Oporto Lisboa
Dr Laura Oporto Lisboa
Dr Beate Ehrhardt and Dr Laura Oporto Lisboa present to colleagues.
Dr Beate Ehrhardt and Dr Laura Oporto Lisboa present to colleagues.
Learn more about Bath research in our monthly email

Machine learning outperforms clinical experts in classifying hip fractures
Hip fractures are a major cause of morbidity and mortality for the elderly and incur high direct health costs. In 2019, 67,671 hip fractures were reported to the UK National Hip Fracture Database and, given the projections for population ageing over the coming decades, the number of hip fractures is predicted to increase globally. Currently, across the world, an estimated 1.6 million hip fractures occur annually with substantial economic burden, approximately $6 billion per year in the US and about £2 billion in the UK.
Accurate assessment and classification of hip fractures can be critical to patient survival. As fracture classification strongly determines the chosen surgical treatment, differences in fracture classification influence patient outcomes and treatment costs. ‘For hip fracture management, the ability to accurately and reliably classify the fracture swiftly is paramount as surgery should occur within 48 hours of admission,’ explains Beate Ehrhardt, Mathematical Innovation Research Fellow. ‘Delays in surgery increase the risk of adverse patients’ outcomes such as mortality.’
Dr Beate Ehrhardt, Mathematical Innovation Research Fellow
Dr Beate Ehrhardt, Mathematical Innovation Research Fellow
However, the increasing demand on radiology departments often means that they cannot report all acquired radiographs in a timely manner. In the UK it is estimated that more than 300,000 radiographs remain unreported for over 30 days. This often means that clinicians are acting on incomplete information when deciding the most-appropriate surgical intervention.
“Machine learning – if used appropriately- can be a powerful tool to support the human in decision making when the amount of input information is overwhelmingly large and there is a large stock of historical data to learn from. Classifying hip fracture from radiographs is an excellent example where mathematics can reduce workload and improve efficiency in clinical workflows.”
Collaborating with Professor Richie Gill in the Department of Mechanical Engineering, Ellen Murphy (Mathematical Innovation Research Associate then and now head of Artificial Intelligence at Jenoptik) and Beate created a machine learning method for identifying and classifying hip fractures, and compared its performance to trained clinical observers. The pair designed, trained, tested and validated two separate convolutional neural networks for automated recognition and classification of hip fractures on radiographs.
Professor Richie Gill
Professor Richie Gill
Working with a panel of medical experts in Bristol Medical School, the North Bristol NHS Trust and the Royal United Hospital NHS Foundation Trust in Bath to validate the results, the algorithm for classifying hip fracture showed an overall accuracy of 92%, compared to human observers who had an accuracy of 77.5% in the original hospital diagnosis.
‘We envisage that this approach could be used clinically and aid in the diagnosis and in the treatment of patients who sustain hip fractures,’ states Beate. ‘What we hope to achieve in the medium term is to test the new approach in an extensive study including multiple NHS hospitals all across the country and our long-term ambition is to implement the new tool in a clinical application to support radiologists in their everyday routine.’
Using data to improve treatment of inflammatory arthritis
Axial Spondyloarthritis (axSpA) is a type of inflammatory arthritis that predominantly affects the axial skeleton causing chronic pain. In the UK, an estimated 220,000 people live with axSpA and, despite the progress made in treatment, there is still potential for improvement.
Dr Dan Burrows, Mathematical Innovation Research Associate, is part of a research group in the IMI to help solve the problem of a ‘one-size-fits-all’ approach to treatment. ‘Data on thousands of patients have been collected to provide information on the effects of different prescribed treatments,’ he says. ‘These measurements carry with them further key insights into the nature of the disease progression.’
Dan, alongside Matthias Ehrhardt and Professor Raj Sengupta from the Department of Mathematics, analysed data from 548 patients to extract patient subgroups based on trends in disease measures. This helps the team to identify shared characteristics in groups with similar response trends, investigate optimal times to initiate a treatment, and predict the disease evolution for a patient, given their current health profile. ‘This approach could allow clinicians to tailor treatment plans more effectively,’ explains Dan.
Dan is hoping that the pilot scheme, funded by the Bath Royal University Hospital (RUH), now leads to a tool that can be used in practice, helping clinicians as a co-pilot for research and decision making. “This could range from a clinician using the tool as an initial exploratory analysis aide, to informing personalised patient treatment by forecasting a patient’s future disease progression and/or identifying optimal treatment interventions,” states Dan.
Dr Dan Burrows
Dr Dan Burrows
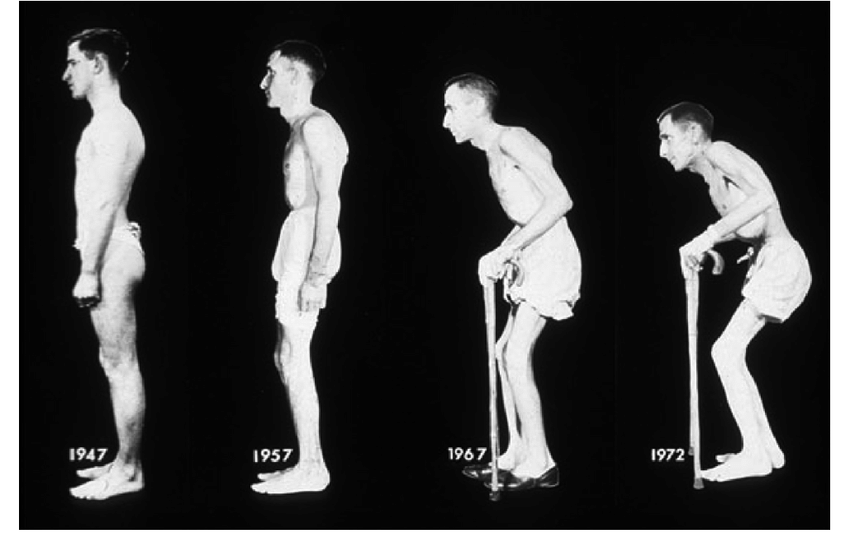
Photographic series of a patient with ankylosing spondylitis taken over a period of 26 years.
Photographic series of a patient with ankylosing spondylitis taken over a period of 26 years.

Research improving support for those who suffer with pain
“What motivates me most is knowing that this work can have real-world impact,” says Laura Oporto Lisboa, Mathematical Innovation Research Associate, whose work focuses on pain. Specifically, how people perceive pain and the wider social and environmental influences of pain.
Dr Laura Oporto Lisboa, Mathematical Innovation Research Associate
Dr Laura Oporto Lisboa, Mathematical Innovation Research Associate
Chronic pain dismantles lives, relationships, and families and, while biological signals can help clinicians understand why pain happens, it does not fully account for the experiences people have, or why pain develops the way it does. Psychological and social factors, such as thoughts and feelings, personal relationships, and lifestyle, can also affect chronic pain.
“We do not yet know which of these psychological and social mechanisms are most important, or how they combine with biological signals to affect chronic pain,” says Laura. “Our aim is to create a clearer account of how, and in what way, psychosocial factors affect pain: what makes chronic pain start, keep going, get better or get worse. “
Professor Ed Keogh
Professor Ed Keogh
Laura, alongside Professor Ed Keogh and several other academic and clinical partners, is part of the Consortium to Research Individual, Interpersonal & Social Influences in Pain (CRIISP), funded by UKRI/Arthritis UK; a multidisciplinary project to better understand how people experience pain and how support can be improved for those who suffer. She is part of a group of analysts working on four large existing longitudinal datasets from people suffering from pain at different stages of life. Her research is helping to create new clinical pathways for pain research, which she hopes will identify ways to prevent chronic pain from happening and reduce the negative effects that pain can have on people's lives.
“People living with chronic pain also have a voice in the whole process. Their experience of what it is like to live with pain is important for us. Together, our work will create new methods for researching pain, new metrics for measuring pain and its effects. We hope to identify crucial psychosocial mechanisms of pain to demonstrate how they interact with biology to either increase or decrease pain.”
Reducing drug development costs
In the drug discovery process, the ability to accurately detect and predict both efficacy and safety relies on the availability of robust models that reproduce, as closely as possible, the effects at patient level. Organs-on-a-Chip are microphysiological systems that aim to recapitulate the complex physiology and microenvironment of an in vivo organ.
The use of Organ-Chips that more accurately recapitulate organ and disease biology have the potential to improve predictability, translatability and in turn, reduce the number of animals needed in drug discovery. However, for these novel organ-on-chip experiments to reach their potential, there is an urgent need for best practices on how to set up the experiment and analyse the data.
Research from Beate Ehrhardt in the Institute for Mathematical Innovation is focused on meeting this need. Working with data scientists from AstraZeneca and lab-on-chip manufacturer Emulate, Beate built a framework of statistical best practice for Organ-Chip studies.
“This research aligns with my passion to enhance reproducibility and translatability of preclinical results across the pharmaceutical sciences with the ambition to reduce reliance on animal testing and lower drug development costs.”
The work, the beginning of a long-term relationship with AstraZeneca, analysed data to create best practice standard guides for the experimental design and analysis of all the Organ-Chip studies at the pharmaceutical company, and Beate hopes her most-recent collaboration on an open-access tool for organ-on-chip experiments will be used across the biomedical research community.
The long-term impact of this project is already becoming clear, and Beate hopes it will be soon adopted by the UK’s national centre for the Replacement, Reduction, and Refinement of animal research (NC3Rs). NC3Rs has launched the DRIVER framework to guide in-vitro research to reduce reliance on animal models. Her work aligns closely with this agenda, offering a principled framework to enhance experimental design and data integrity in in-vitro experiments.


Our research is helping to improve the world. Through collaborative partnerships we're creating a healthier, more sustainable, and connected future for all.