Why tomorrow's vaccines will come with a pinch of sand

Technology to make vaccines stable in all temperatures by encasing them in silica – simple sand – is being developed by chemists at the University of Bath.
This ground-breaking solution to the problem of vaccines 'spoiling' means more people around the world can be saved from vaccine-preventable diseases.
What do smallpox and cattle plague (rinderpest) have in common?
Both have the potential to cause deadly infections (one in humans, the other in cattle), both are highly transmissible and both are problems of the past.
But perhaps most significantly, both diseases have been eradicated by vaccines that can be stored at room temperature. And this makes them almost unique.
Virtually all other vaccines (with the exception of polio, which is also close to being wiped off the planet) require refrigeration to stop them from going off. From the moment they’re sealed in a vial to the moment they’re injected into living tissue, these life-saving formulations need to be kept cold – sometimes frozen – to safely and effectively inoculate against infection.
And here’s the rub: keeping vaccines cold is no mean feat. The journey, from the factory where they’re produced to the living creature they’re intended for, is often long and twisted, and all too frequently failures occur along the way. The moment there’s a bump in the road – sometimes quite literally – and temperatures crank up, a vaccine ‘spoils’ and can no longer be used. Above 20°C, some vaccines don’t last more than an hour.
This could all change, however, thanks to the ground-breaking work of researchers at the University of Bath, led by visionary chemist Dr Asel Sartbaeva.


"Our technology promises to be a game-changer for families all over the world – especially those living in remote and impoverished areas."

After a decade of dedicated lab work, Dr Sartbaeva and her team have found a way to keep the molecular structure of most vaccines intact without the need for refrigeration.
How?
By wrapping a vaccine’s active ingredient in an inert shell that prevents parts of the structure sticking together and also stops the structure as a whole from unravelling. Clumping and unfolding are the main two reasons vaccine proteins deteriorate when they’re removed from cold storage.
Using the Bath team’s award-winning, patented technology – named ensilication – a vaccine can now be warmed to boiling-point temperatures and jiggled about in extreme humidity without falling apart.
Dr Sartbaeva is confident that ‘ensilicated’ vaccines will be available for use in animals within just two years and in humans, possibly, within five.
Vaccine inequity
Vaccines really matter. Annually, they prevent 3.5–5 million people around the world from dying, either by inhibiting infection or by reducing its severity. Currently, vaccines exist for 25 life-threatening diseases, including measles, influenza, whooping cough and, of course, Covid-19. Fifteen more are in the pipeline.
Yet, every year 1.5 million children miss out on vaccines. Globally, three million children under five die because they’re not receiving routine inoculations (and in 2020, the year Covid-19 took hold, a staggering 23 million children under the age of one year did not receive basic vaccines). The vast majority of these children live in low-income countries. This inequity in vaccine availability is spurring on Dr Sartbaeva and her team.
"This needs to change. Immunisation is a basic human right and finding ways to deliver life-saving vaccines to all corners of the world should be a global priority."

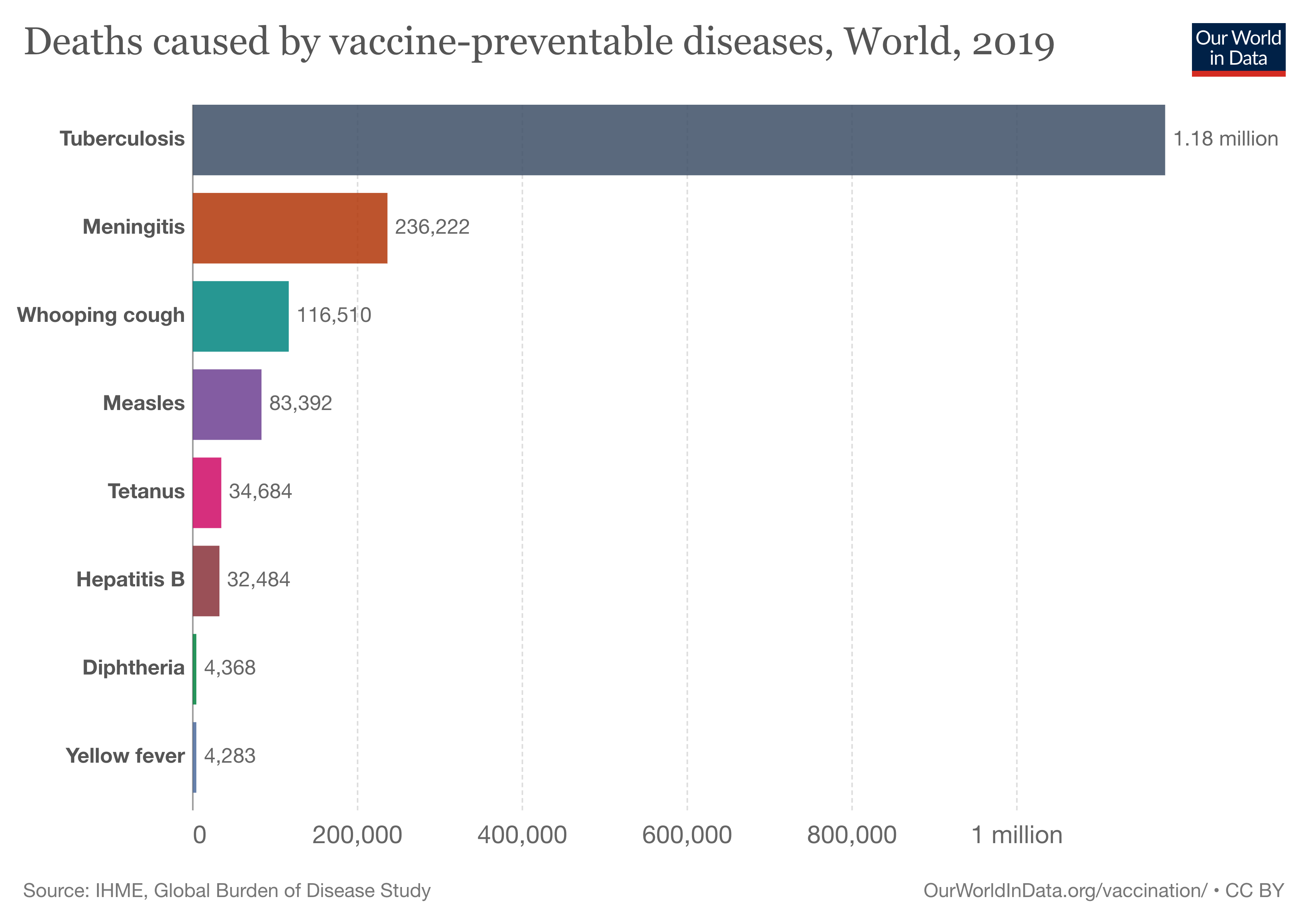
The chilling story of vaccine transportation
The active ingredient of most 'traditional' vaccines is a protein, and it is this element that requires refrigeration to stay intact. Generally, a temperature of 2-8°C will do the trick, but sometimes – as is the case for the Pfizer-BioNTech Covid-19 vaccine (made with new mRNA technology) – the dial must be turned way down, to -70°C, for the active ingredients to hold together.
Delivering vaccines that need to be kept cold to communities riven by poverty or conflict – or both – is a logistical nightmare. Take the example of a measles vaccine dispatched from Europe to a remote corner of Nepal. To reach its destination, it must travel by refrigerated truck from a factory in its country of origin to an airport, then by refrigerated airplane to its destination country, then again by refrigerated truck to a remote clinic, and finally by refrigerated van or boat – or perhaps in a cool box hitched to a horse, camel, bike or a person’s back – to an isolated village, where it must be stored in chilled or even freezing conditions until the time comes for it to be administered.
With such complex logistics, it’s easy to see how at some point in the journey, the cold chain might be broken. And though waste through mishaps in refrigeration isn’t the sole reason people miss out on life-saving vaccines, breaks in the cold chain are a serious global public health issue – and costly too.








WHO / Jawad Jalali
WHO / Jawad Jalali

WHO / Blink media - Uma Bista
WHO / Blink media - Uma Bista

WHO / Olivia Acland
WHO / Olivia Acland

WHO / Thomas Moran
WHO / Thomas Moran

WHO / Ilyas Ahmed
WHO / Ilyas Ahmed

WHO / Henrietta Allen
WHO / Henrietta Allen
For good reason, thermostable vaccines were named a priority research area in the World Health Organisation’s Global Vaccine Action Plan 2011-2020.
A technical specialist at UNICEF has assured Dr Sartbaeva that a technique allowing cold chain to be eliminated from vaccine transportation and storage would be ‘transformative’.
UNICEF, the biggest distributor of vaccines worldwide, procured more than US$103 million (£84 million) worth of cold-chain equipment and services in 2020. According to research from Imperial College London, cold-chain processes account for up to 80% of the costs of a vaccination programme.
"Half of all vaccines end up being thrown away, and this is largely because of the length and intricacy of many journeys – it’s a terrible waste."
Tiny vaccine cage
The secret to keeping vaccines intact at room temperature is to envelop them in silica – simple sand, like beach sand, only cleaner. Silica is inorganic (it contains no elementary carbon), non-toxic and biocompatible, meaning it’s not harmful to human tissue – indeed, the FDA has approved it for oral delivery (which perhaps should come as no surprise – children often eat sand and do just fine). It’s also cheap and readily available.
"The fundamental science behind ensilication is astonishingly simple."
The first step in the ensilication process involves breaking silica down into its most elementary chemical unit: one silicon atom bonded to four oxygen atoms. In nature, silica never presents this way, preferring instead to form a giant, super-stable 3D molecular framework with many silicon-oxygen units bonded together.
“Silica will always try to be in this large, polymeric, 3D state,” explains Dr Sartbaeva. “Discovering a technique to keep the molecules separated was a challenge – they need to be sitting in a medium that's kept at a specific pH, and you need to move fast with the second step in the process.”


Putting it all together
That second step involves adding the tiny, standalone silica molecules to a solution of vaccine protein. This instantly causes the silica to start clumping again to reform its preferred supersized structure. Where a protein molecule is in the way of this re-bonding process, the silica molecules simply wrap around it, using the protein as a template at the centre of their newly formed structure, effectively trapping it in a silica polymer shell.
When this step is complete, the new compound is filtered to remove the liquid, and what remains is a dry vaccine-silica powder that is stable and safe, and can be stored and transported without refrigeration.

From sand to vaccine: Ensilicated vaccine powder is what remains after the fluid medium has been filtered out.
From sand to vaccine: Ensilicated vaccine powder is what remains after the fluid medium has been filtered out.
"The vaccine can no longer break down,” explains Dr Sartbaeva. “Not only is it indifferent to heat but it also has a long shelf life of at least three years with no loss of function."
Rolling it out
Having mastered ensilication, the team is now working on a method for separating the protein from its silica cage in the moments before a vaccine is administered. This will involve ‘cracking’ the shell to return the silica to its simplest form then filtering the silica molecules from the larger protein molecules (Dr Sartbaeva believes she’s close to mastering a technique). Next will come human trials and finally the necessary regulatory steps.
“After that, we hope we’ll be able to roll this technology out,” says Dr Sartbaeva.

Shifting sands
The spark for her big research idea came 12 years ago when Dr Sartbaeva – who completed her undergraduate degree in her home country of Kyrgyzstan – found herself in a doctor’s surgery in Oxford while her baby daughter received her BCG jab for tuberculosis, the first of many childhood vaccines.

Dr Asel Sartbaeva and her fully vaccinated daughter
Dr Asel Sartbaeva and her fully vaccinated daughter
“The injection was prepared from a vial pulled from a fridge, and that just got me thinking – why do vaccines need to be kept cold? And is there anything we can do to change this? At the time, I knew very little about vaccines, so I went away and learnt everything I could. It took about two years for me to get a firm grip on the basic science of what vaccines are made of, and to develop an idea for what I’d do.
Before setting out on her quest to stabilise vaccines, Dr Sartbaeva worked with zeolites – a large group of minerals commonly used as catalysts in industrial processes, though also of interest in medicine. “I loved this work, but vaccines became my obsession,” she says.
In 2010 she accepted a Royal Society Fellowship for her work on zeolite-related research. The Fellowship moved with her to the University of Bath in 2012. At Bath, her mentor Professor Paul Raithby was keen for her to explore ensilication on the side. He introduced her to Professor Jean van Den Elsen, who set her on her path.
“Jean said, ‘Here is a protein, start working on it!’ Another colleague – Professor Karen Edler – said, ‘Here is some lab equipment, let’s collaborate’. Suddenly, I was in a position to start doing exciting stuff in the lab, working on an idea that had been completely theoretical in my head until that moment.”
She adds: “I can honestly say that ensilication would have remained a curious ‘what if?’ if it hadn’t been for the incredible atmosphere of collaborations at Bath, along with the availability of money to fund the research, and a number of excellent PhD students who I was able to recruit for this project.”
Vaccines everywhere all at once

After an initial flurry of publicity for her project, Dr Sartbaeva lurched from one failed experiment to the next for several years. She simply couldn’t crack the code for ‘sealing’ a temperature-sensitive protein in a capsule that would stop it from decaying.
Then in 2014, her luck began to turn. She was contacted by Google X – the brainstorming arm of the tech giant – and invited to present her ensilication dream in Los Angeles at a conference aimed at ‘techies’ and entrepreneurs.
“Talking about my idea at this conference made me very uncomfortable,” she remembers. “As scientists, we present concrete results and we have to be able to defend our research findings against severe criticism and people looking to pull your work apart. I only had an idea at that stage, no concrete results, data or any findings.”
But despite her misgivings, she spoke at the conference and, to her amazement, the audience was hugely encouraging. She returned to her lab with a spring in her step.
“I started approaching the work with a fresh mindset and, unbelievably, our experiments started working the very week after my return. I kept checking and rechecking the results – it didn’t seem possible. But things really were heading the right way.”
A second watershed moment came in 2015, when Dr Sartbaeva hired (now Dr) Aswin Doekhie on a mature studentship. After studying biology as an undergraduate, Dr Doekhie had spent his early career working in biopharma. “He brought a set of knowledge that I was lacking at the time – we needed a biologist and he was the perfect fit.”
By the time Dr Doekhie had finished his PhD in 2019, the ensilication team – now numbering six – was getting concrete results on tetanus ensilication, and had submitted its first of three patent applications.
Animal trials followed, with the researchers sending both ensilicated and regular samples of the tetanus vaccine from Bath to Newcastle by ordinary post (a journey time of over 300 miles, which by post takes a day or two) and subsequently injecting doses into mice.
“An immune response was triggered with the ensilicated samples, showing the vaccine to be active, but no immune response was detected in mice injected with unprotected doses of the vaccine, showing the medicine was damaged in transit,” explains Dr Doekhie.
Then came Covid-19 and the team’s research was shot with a whole new sense of urgency.
Vaccine wastage in a pandemic
“Before the pandemic, it was clear that some people in the biopharma industry – the people who make vaccines – wouldn’t be interested in our technology,” says Dr Sartbaeva. “After all, they make money from 50% of vaccines going to waste. It was disheartening. But during the pandemic, vaccine wastage became very topical, and suddenly the conversation changed. Companies started saying: ‘We need your technology – talk to us.’”


This change in tone – along with Sartbaeva’s win of the prestigious Emerging Technology Competition by the Royal Society of Chemistry in 2020 and the FDM everywoman in Technology Award in 2021 – persuaded the team to launch EnsiliTech, “to take the technology out of the lab and into the world.”
The spinout company, now incorporated, has received an Innovate UK grant to help get it off the ground. Dr Sartbaeva is CEO and Dr Doekhie CTO.
Our mission is to make vaccines available to everyone worldwide, more cheaply than they are now, and to eradicate all vaccine-preventable diseases. Our vision is to apply ensilication to as many targets as possible – not just vaccines but also proteins used to diagnose disease (think lateral flow tests) and monoclonal antibodies, which are used to treat disease and which now also need to be kept extremely cold.”
The hope is that ensilicated proteins will eventually come in tablet form too, to remove just one more obstacle to vaccination – a fear of needles.
"Using ensilication to reduce child mortality and improve global health and wellbeing would be a dream come true for the entire team."

Research papers
Thermal stability, storage and release of proteins with tailored fit in silica Chen, Y-C., Smith, T., Hicks, R. H., Doekhie, A.,Koumanov, F., Wells, S. A., Edler, K. J., van den Elsen, J., Holman, G. D., Marchbank, K. J.& Sartbaeva, A., 24 Apr 2017: Nature Scientific Reports. 7,p. 1-88 p., 46568.
Ensilication Improves the Thermal Stability of the Tuberculosis Antigen Ag85b and an Sbi-Ag85b Vaccine Conjugate Wahid, A., Doekhie, A., Sartbaeva, A. & Van Den Elsen, J. 8 Aug 2019: Nature Scientific Reports. 9,1, 11409.
Ensilicated tetanus antigen retains immunogenicity: in vivo study and time-resolved SAXS characterization Doekhie, A., Dattani, R., Chen, Y-C., Yang, Y., Smith, A., Silve, A. P., Koumanov, F., Wells, S., Edler, K., Marchbank, K. J., Van Den Elsen, J. & Sartbaeva, A.,1 Dec 2020: Nature Scientific Reports.10,1, 9243.
Thermal resilience of ensilicated lysozyme via calorimetric and in vivo analysis Doekhie, A., Slade, M., Cliff, L., Weaver, L., Castaing, R., Paulin, J., Chen, Y-C., Edler, K., Koumanov, F., Marchbank, K., Van Den Elsen, J. & Sartbaeva, A.,12 Aug 2020: RSC Advances.10,50,p. 29789-297968 p.
Physiochemical changes to TTCF ensilication investigated using time-resolved SAXS Doekhie, A., Dattani, R., Chen, Y-C., Koumanov, F., Edler, K., Van Den Elsen, J. & Sartbaeva, A.,5 Aug 2021: AppliedChem.1,1,p. 4-1310 p.

